Discover a comprehensive step-by-step guide to the hot dip galvanizing process. From surface preparation to immersion in the molten zinc bath, our detailed explanation covers every crucial stage. Learn about fluxing, alloying, withdrawal, cooling, and inspection to ensure a high-quality zinc coating. Explore the benefits of this corrosion-resistant technique and gain insights into best practices for successful galvanization. Whether you’re new to the process or seeking a deeper understanding, our guide provides valuable knowledge to help you master hot dip galvanizing.

Hot-dip galvanizing is a process used to apply a protective coating of zinc to steel or iron surfaces. It involves immersing the cleaned and prepped steel or iron articles into a bath of molten zinc at a temperature of approximately 450 to 460 degrees Celsius (850 to 860 degrees Fahrenheit).
The process of hot-dip galvanizing consists of three key steps: surface preparation, galvanizing, and inspection. They are shown in the hot dip galvanizing process diagram below:
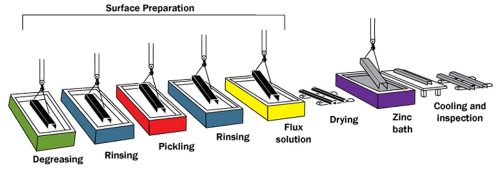
The hot dip galvanizing process diagram
The resulting zinc coating provides a durable, corrosion-resistant barrier that protects the steel or iron from rust and other environmental factors. Hot-dip galvanizing is widely used in various industries, including construction, automotive, infrastructure, and manufacturing, to extend the lifespan and enhance the performance of metal products.
Surface preparation is an important step in applying any coating. The majority of coating failures occur before the end of their anticipated service life due to improper or insufficient surface preparation.
Because zinc will not react with dirty steel, the surface preparation phase in the galvanizing process has its own built-in quality control. Any flaws or deficiencies in surface preparation will be instantly visible when the steel is removed from the zinc bath because the unclean regions will remain uncoated, allowing for prompt corrective action.

Three steps are involved in surface preparation:
Organic pollutants such as dirt, paint marks, grease, and oil are removed from the metal surface using a hot alkali solution, mild acidic bath, or biological cleaning bath. Epoxies, vinyls, asphalt, or welding slag that cannot be removed by degreasing must be removed by grit-blasting, sand-blasting, or other mechanical means before galvanizing.
Mill scale and iron oxides (rust) are removed from the steel surface using a dilute solution of hot sulfuric acid or ambient hydrochloric acid. In addition to or instead of pickling, this stage can be completed by abrasive cleaning or air blasting sand, metallic shot, or grit onto the steel.
The cleaned articles are dipped into a flux solution or passed through a fluxing chamber. The flux removes any remaining oxides from the surface and provides a protective layer on the steel, promoting the formation of a uniform zinc coating.
Depending on the size and intricacy of the objects to be galvanized, the preparation process might take anywhere from a few minutes to an hour. Small pieces, for example, can be cleaned and prepared quickly, whereas massive structures, such as I-beams and girders, can take up to an hour to prepare.
When steel is entirely immersed in a bath (kettle) of molten zinc, the galvanizing step of the process happens. The bath chemistry must be at least 98% pure zinc and kept at a temperature of around 850 °F(450 °C), according to the specifications. The crane hoist lowers the steel at an angle. This allows air to escape from tubular shapes or pockets in the design of a fabricated component, as well as molten zinc to displace the air.
The zinc reacts with the iron in the steel in the kettle to generate a sequence of zinc-iron intermetallic alloy layers. The coating growth is complete after the fabricated item reaches bath temperature, and the products are slowly removed from the galvanizing bath. Draining, vibrating, and/or centrifuging are used to remove excess zinc. As long as the piece remains around bath temperature after being removed from the bath, the metallurgical reaction will continue. Articles are cooled either by immersing them in a passivation solution or by leaving them out in the open air.

The most time-consuming component of the endeavor is the preparation. When the preparatory procedure is finished, the steel or iron products are ready for hot-dip galvanizing.
As previously said, hot-dip galvanizing takes only minutes, however this time can vary depending on the size and shape of the products being galvanized. Small pieces can be galvanized in a matter of minutes, however larger structures may require up to an hour to fully submerge and remove from the galvanizing bath.
The final stage of the procedure, inspection, is straightforward and rapid. Coating thickness and coating look are the two aspects of the hot-dip galvanized coating that are thoroughly examined. Additional tests for adherence are outlined, however these are normally only administered as a referee test or when an issue is suspected.
A visual assessment of the material can provide a highly precise determination of the quality of the galvanized coating since, as previously indicated, zinc will not react with filthy steel. A range of basic physical and laboratory tests may also be done to check that the coating meets specification requirements for thickness, homogeneity, adhesion, and appearance. Products are galvanized in accordance with ASTM standards that have been long established, accepted, and approved.

During the hot-dip galvanizing process, there are several important factors to keep in mind to ensure a successful and high-quality coating. Here are some key considerations:
Cleaning Methods: To effectively remove impurities from the surface, use effective cleaning processes such as degreasing, alkaline cleaning, acid pickling, or abrasive blasting. The cleanliness of the surface has a direct impact on the zinc coating’s quality. Because Poor adhesion and coating flaws might result from insufficient surface preparation.
Material Selection: Ensure that the steel or iron being galvanized is suitable for the process. Certain materials, such as cast iron or steels with high silicon or phosphorus content, may require special considerations or pre-treatments.
Surface Profile: Achieve an appropriate surface profile through abrasive blasting or other mechanical methods. A suitable surface profile promotes better zinc adhesion and coating durability.
Material Thickness: Consider the thickness of the steel or iron articles, as thicker materials may require longer immersion times to achieve the desired coating thickness.
Design Considerations: Ensure that the design of the articles allows for proper drainage of excess zinc and uniform coating thickness. Avoid sharp edges, crevices, or complex geometries that can trap zinc or hinder drainage.

Flux Selection: Choose the appropriate flux formulation based on the specific requirements of the galvanizing process and the type of steel or iron being galvanized. Different fluxes are available for different applications and materials.
Bath Management: Regularly monitor and maintain the composition and temperature of the zinc bath to ensure consistent and optimal galvanizing conditions. This includes periodic analysis of the zinc bath to determine the need for replenishment or adjustments.
Immersion and Withdrawal Speed: Control the immersion and withdrawal speed of the articles to allow for proper coating formation and drainage. Avoid rapid or jerky movements that can cause coating defects or uneven thickness.
Post-Galvanizing Treatment: Consider any post-galvanizing treatments that may be required, such as chromate passivation or topcoat application, to enhance the appearance or further improve corrosion resistance.
Handling and Packaging: Handle galvanized articles with care to avoid scratching or damaging the coating. Proper packaging and storage techniques can help prevent coating damage during transportation or storage.
Quality Assurance: Implement a robust quality control program that includes regular inspections, testing, and adherence to relevant industry standards and specifications. This helps ensure that the galvanized articles meet the desired quality requirements.
By paying attention to these detailed considerations, you can optimize the hot-dip galvanizing process to achieve superior corrosion protection and coating quality for your steel or iron articles.
Learn more
Hot Dipped Galvanized Steel Coils: What You Should Know
Top 10 Domestic and Global Galvanized Steel Coil Suppliers
Galvanized Steel Coils: Essential Information You Need to Know
In conclusion, understanding the step-by-step process of hot dip galvanizing is essential for anyone involved in the fabrication or use of galvanized steel or iron. By following proper surface preparation, fluxing, immersion, withdrawal, and cooling techniques, a high-quality zinc coating can be achieved, providing excellent corrosion resistance and durability. Attention to detail, such as material selection, drainage, and post-galvanizing treatments, further enhances the effectiveness of the process. With this comprehensive knowledge, you can confidently navigate the hot dip galvanizing process, ensuring the longevity and performance of your galvanized articles in diverse applications across various industries.
Address
Website: https://stavianmetal.com
Email: info@stavianmetal.com
